Derived mean values of the specific heat of pure iron in comparison... | Download Scientific Diagram

Calculate the amount of heat required to raise the temperature of 5 g of iron from `25^(@)C \"to\" - YouTube

Comparison between the calculated and experimental heat capacity of... | Download Scientific Diagram

A piece of iron of mass 100g is kept inside a furnace for a long time and then put in a calorimeter of water equivalent 10g containing 240g of water at 20^oC .
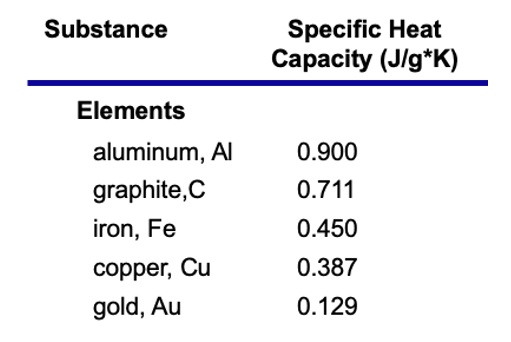
SOLVED: Text: Substance Specific Heat Capacity (J/g*K) Elements: aluminum, Al; graphite, C; iron, Fe; copper, Cu; gold, Au 0.900 J/g*K, 0.711 J/g*K, 0.450 J/g*K, 0.387 J/g*K, 0.129 J/g*K

Color online) Temperature-dependent specific heat capacities of (a)... | Download Scientific Diagram